Published on September 27, 2018
September 27, 2018
By Carole Baggerly
Last week, I was at the FDA in Washington, DC to present a health claim petition about the effect of vitamin D on preterm birth prevention, with Dr. Newman from the Medical University of South Carolina (MUSC) and the Organic and Natural Health Association. The FDA informed us they had laws that required that the intake amount of a supplement had to be correlated with the health outcome. However, the petition being submitted made the association with the blood serum level (25(OH)D), which requires measurement. As most of our readers know, the association is not with the intake but with the serum level. This week, upon returning to MUSC, Dr. Newman sent out this note to the Obstetrics clinic and staff:
 “This is the cover Editorial from The Endocrine Society and discusses a study by Rostami et al J Clin Endocrinol and Metab 2018 reporting on perinatal benefits of screening and supplementing pregnant women who are Vitamin D deficient (large majority of our patients). In that study they reduced pre-eclampsia by 60%; Gestational Diabetes by 50% and Preterm Births by 40%. This concept is exactly what we initiated in 2015 in our clinics based on Carol Wagner’s RCT (again MUSC ahead of the curve). We have already published on our significant reductions in PTB in over 1000 women with our Field Trial with GrassrootsHealth; Julio Mateus at North Carolina has an abstract at the Society of Maternal and Fetal Medicine about our sign reductions in pre-eclampsia and we haven’t looked at Gestational Diabetes yet.
“This is the cover Editorial from The Endocrine Society and discusses a study by Rostami et al J Clin Endocrinol and Metab 2018 reporting on perinatal benefits of screening and supplementing pregnant women who are Vitamin D deficient (large majority of our patients). In that study they reduced pre-eclampsia by 60%; Gestational Diabetes by 50% and Preterm Births by 40%. This concept is exactly what we initiated in 2015 in our clinics based on Carol Wagner’s RCT (again MUSC ahead of the curve). We have already published on our significant reductions in PTB in over 1000 women with our Field Trial with GrassrootsHealth; Julio Mateus at North Carolina has an abstract at the Society of Maternal and Fetal Medicine about our sign reductions in pre-eclampsia and we haven’t looked at Gestational Diabetes yet.
But this is why we need to
- Screen for Vitamin D deficiency (less than 40 ng/ml (100 nmol/L);
- Identify it when present;
- Supplement aggressively if deficient (free samples of 5000IU/day are available to anyone) and
- (maybe most important) Follow up at 24-28 weeks to see if levels have risen with a goal of > 40 ng/ml
Besides compliance, there is a very non-linear dose response curve and some women will require up to 10,000 IU daily to move the needle.
Last week I spoke at an FDA meeting in Washington DC (BTW- that is where Hurricane Florence went) about adding PTB prevention as a labeling indication for Vitamin D supplements. They (FDA) aren’t ready to bite on that just yet-but they were very interested in the evidence – especially outcomes associated with increasing levels to >40 ng/ml- and the day is coming soon where they will.
The evidence is accumulating and they need to think outside the box and look at achieved serum Vitamin D levels vs intent to treat studies with insufficient dosing. The day is coming and we will have been there first.
Thanks and keep working to keep our women successfully Vitamin D supplemented.”
Dr. Newman has taken the initiative since the beginning to ‘move research into practice’, worked with us closely to monitor both scientific and personnel performance, and to further the cause amongst the profession. He is a beautiful example of ‘what it takes’—knowledge, dedication, and action! We commend Dr. Newman as being a LEADER of the Protect our Children NOW! movement. Thank you, Dr. Newman, for the women who will benefit from your involvement and a special thanks from the professional community as well.
Please see the links below for more information about the research and other information.
Have a healthy LIFE!
Carole
Should Pregnant Women be Screened for Vitamin D Deficiency?
A recent publication by Rostami et al reported on the benefits of vitamin D screening and supplementation for pregnant women. Not only did screening allow the majority of women to correct pre-existing vitamin D deficiency, which has been shown to have negative effects on both the health of the pregnancy and the newborn, they observed a significant decrease in adverse prenatal outcomes. For the women who were screened, a 60% decrease in preeclampsia, a 50% decrease in gestational diabetes mellitus, and a 40% decrease in preterm delivery was observed.
This is Not New News!
In 2015, GrassrootsHealth, with the help of Dr. Roger Newman, initiated a new standard of care for prenatal women at the Medical University of South Carolina (MUSC) to screen all pregnant women for vitamin D levels and provide them with supplements to help get their levels up to at least 40 ng/ml.
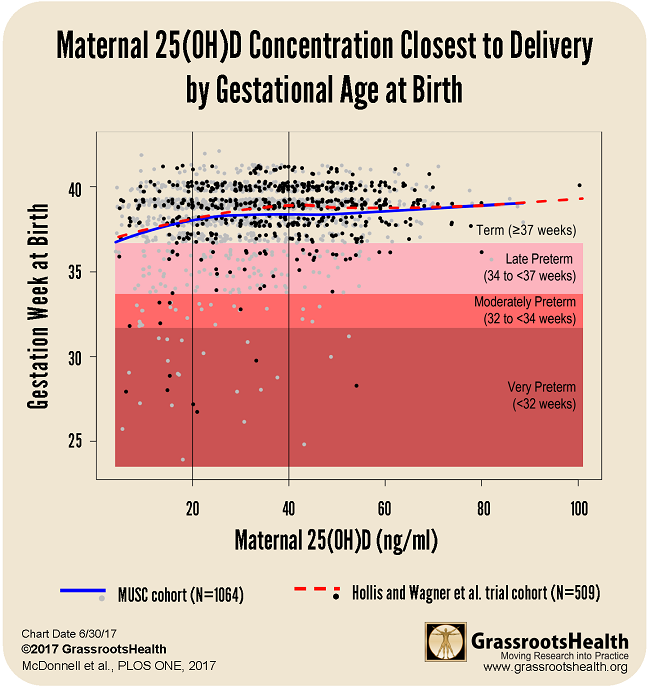
Overall, women who got their vitamin D level above 40 ng/ml (100 nmol/L) by the time of delivery had a 62% lower risk of preterm birth as compared to women with levels under 20 ng/ml (50 nmol/L).
Additional Evidence through Repeated Findings
The findings from the field trial at MUSC align closely with previous analyses of randomized controlled trials on vitamin D and preterm birth outcomes. One of these studies showed a 59% lower preterm birth risk when comparing the same serum level groups (≥ 40 ng/ml vs. <20 ng/ml) (Post-hoc Analysis of Vitamin D Status and Reduced Risk of Preterm Birth in Two Vitamin D Pregnancy Cohorts Compared with South Carolina March of Dimes 2009-2011 rates, Wagner et al., November 2015).
Below is a chart that shows results from both the MUSC cohort (N=1,064) and the Hollis and Wagner et al. RCT cohort (N=509) with confidence bounds. In both sets of data, there was a steady increase of gestation time correlating to the rise of the vitamin D serum level up to a plateau around 40 ng/ml.
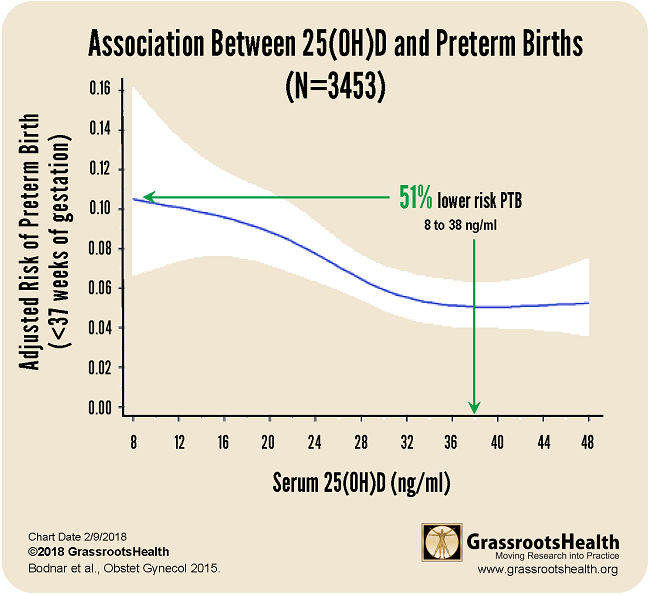
Results published in a paper by Bodnar et al., Early-pregnancy vitamin D deficiency and risk of preterm birth subtypes, demonstrated similar findings among a group of 3,453 pregnant women. For this cohort, there was a 51% lower risk of preterm birth for women with a vitamin D serum level of 38 ng/ml or greater, compared to women with 8 ng/ml.
Are You or Someone You Know Newly Pregnant?
Share this important information with them, and suggest they enroll in the Protect Our Children NOW! online program for free, at-home vitamin D testing and supplementation.
