Published on January 16, 2019
How can we interpret the vastly different reports about the VITAL Randomized Controlled Trial of vitamin D and omega-3? Is there truly “…no lower incidence of major invasive cancer or cardiovascular events than placebo” as reported by some news articles?
The purpose of this blog is to answer two primary items:
- SHOUT from the ROOFTOPS the PUBLIC HEALTH ACTIONS that need to be taken now on the 30 significant outcomes from the secondary analyses: make sure the omega-3 index test is done and everyone gets enough omega-3 and, do vitamin D testing along with appropriate intake to achieve at least 40 ng/ml (100 nmol/L).
- Explain how the focus with the research release of this study focused on ONLY the so-called ‘primary outcomes’ vs ALL analyses.
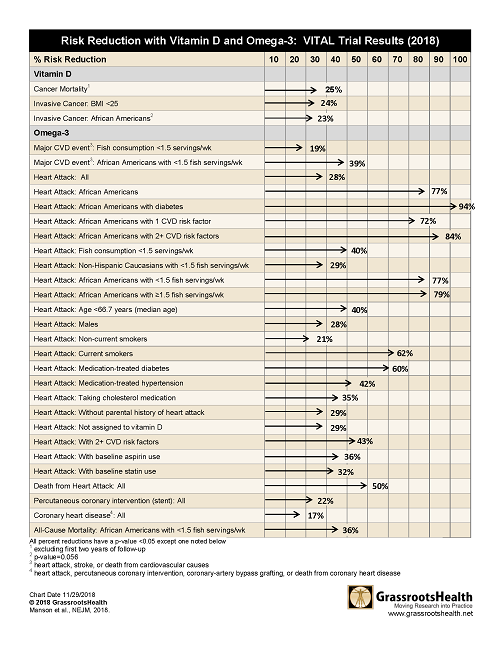
What choices would you make about your omega intake and vitamin D given the following results which showed
- 50% reduction in death from heart attack for all
- 77% reduction in heart attack for African Americans
- 25% reduction in risk of cancer mortality
- ….many others, see below
 Why the shouting?
Why the shouting?
- Vitamin D has a demonstrated safety profile – it is READY to be implemented NOW!
- It is easily available to all
- Very low cost ($0.05/day for supplements)
- The BENEFITS are enormous for taking action now
WE CAN’T WAIT for ongoing trials to get more information to start taking advantage of this health benefit. We shouldn’t wait!
Just take a look at these results and decide if you think it’s worth the wait:
Explanation of RCT process
Most clinical trials examine many outcomes during the course of a study. During the design phase of the study, primary and secondary outcomes are specified along with specific sub-group analyses. The primary outcome is considered the most important measure to evaluate the effect of a particular intervention (this choice is made by the scientists). The primary outcome is used as a guide to determine how many people to enroll in the study and how long it should last.
Secondary outcomes are measures that are of interest, and may support the primary outcome, but because the trial was not designed for these outcomes there may not be the same certainty in results as the primary outcome. Pre-specifying outcomes reduces the risk of finding false-positive results because the more outcomes you test, the more likely you are to find a significant result by chance alone.
The VITAL trial, which focused on cancer and cardiovascular health, recently published two results papers: one paper comparing those assigned to vitamin D (2000 IU/day) vs. placebo and another paper comparing those assigned to omega-3s (1000 mg/day) vs. placebo. You may have read in the news that this study showed that neither vitamin D nor omega-3s prevent cancer (all kinds of cancer analyzed together) or heart disease (a composite of all types).
However, when the separate types of heart disease or death from cancer were analyzed, there were 30 different very significant results. See the tables below or download, print and share here.
Statistically Significant Vitamin D Results from Pre-specified Secondary Outcomes and Subgroup Analyses
|
Vitamin D vs. Placebo
|
|
|
Secondary Outcomes
|
|
|
Cancer Mortality
(excluding first two years of follow-up)
|
25% reduced risk (p=0.02)
|
|
Primary Outcomes – Subgroup Analyses
|
|
|
Invasive Cancer
|
|
|
BMI<25
|
24% reduced risk (p=0.003) |
|
African Americans
|
23% reduced risk (p=0.056*) |
Statistically Significant Omega-3 Results from Pre-specified Secondary Outcomes and Subgroup Analyses
|
Omega-3 vs. Placebo
|
|
|
Secondary Outcomes
|
|
|
Heart Attack
|
28% reduced risk (p=0.002)
|
|
Primary Outcomes – Subgroup Analyses
|
|
|
Major CVD event
(heart attack, stroke, or death from cardiovascular cause)
|
|
|
Fish consumption <1.5 servings/wk
|
19% reduced risk (p=0.03)
|
|
African Americans with <1.5 servings of fish/wk
|
39% reduced risk (p=0.049)
|
|
Secondary Outcomes – Subgroup Analyses
|
|
|
Heart Attack
|
|
|
African Americans
|
77% reduced risk (p=<0.0001)
|
|
African Americans with diabetes
|
94% reduced risk (p=0.005)
|
|
African Americans with 1 cardiovascular risk factor
|
72% reduced risk (p=0.047)
|
|
African Americans with 2+ cardiovascular risk factors
|
84% reduced risk (p=0.001)
|
|
Fish consumption <1.5 servings/wk
|
40% reduced risk (p=0.0007)
|
|
Non-Hispanic Caucasians with <1.5 servings of fish/wk
|
29% reduced risk (p=0.04)
|
|
African Americans with <1.5 servings of fish/wk
|
77% reduced risk (p=0.003)
|
|
African Americans with ≥1.5 servings of fish/wk
|
79% reduced risk (p=0.01)
|
|
Age <66.7 years (median age)
|
40% reduced risk (p=0.005)
|
|
Males
|
28% reduced risk (p=0.02)
|
|
Non-current Smokers
|
21% reduced risk (p=0.04)
|
|
Current Smokers
|
62% reduced risk (p=0.02)
|
|
Medication-treated diabetes
|
60% reduced risk (p=0.0003)
|
|
Medication-treated hypertension
|
42% reduced risk (p=0.0002)
|
|
Taking cholesterol medication
|
35% reduced risk (p=0.02)
|
|
Without parental history of heart attack
|
29% reduced risk (p=0.008)
|
|
Not assigned to vitamin D
|
29% reduced risk (p=0.02)
|
|
With 2+ cardiovascular risk factors
|
43% reduced risk (p=0.001)
|
|
With baseline aspirin use
|
36% reduced risk (p=0.007)
|
|
With baseline statin use
|
32% reduced risk (p=0.04)
|
|
All-Cause Mortality
|
|
|
African Americans with <1.5 servings of fish/wk
|
36% reduced risk (p=0.03)
|
Researchers can also publish results on outcomes that were not pre-specified (i.e. post hoc), but these results are considered preliminary because they are typically added once a pattern has been discovered in the data and therefore have a higher false-positive risk. The VITAL trial reported a few statistically significant post hoc tests for those assigned to omega-3 vs. those assigned to placebo (see table below).
Statistically Significant Omega-3 Results from Post-Hoc Outcomes
|
Omega-3 vs. Placebo
|
|
|
Post-Hoc Outcomes
|
|
|
Death from Heart Attack
|
50% reduced risk (p=0.04)
|
|
Percutaneous coronary intervention (PCI; stent)
|
22% reduced risk (p=0.02)
|
|
Coronary heart disease (heart attack, PCI, CVD death)
|
17% reduced risk (p=0.02)
|
A printable version of these results can be downloaded here.
The effects from vitamin D were not as prolific as those from the omega-3’s likely due to the relatively low dose of vitamin D given (2000 IU/day). To get to a vitamin D serum level of 40 ng/ml (100 nmol/L), our scientists’ panel’s recommendation, it would take 5000 IU/day to get approximately 82% of the population to that level. Also, the data from this study were not analyzed by serum level, so the results of higher and lower levels would be averaged and miss the educational point of the serum level significance.
Given the significant positive effects of vitamin D on cancer death and omega-3 on cardiovascular health found in the VITAL trial (which is supported by many other studies), and the fact that these supplements are safe and inexpensive, daily supplementation is beneficial. Further, vitamin D and omega-3 blood level testing should be used to personalize intake amount since there is a large range of variability in the response to a particular dose.
Please SHOUT the results and actions about various intakes to friends, everyone you meet-we have a health crisis that can be significantly averted with ONLY vitamin D and/or Omega 3!!!
Onwards for PUBLIC HEALTH! from GrassrootsHealth,
Sharon McDonnell
Senior Statistician
Jen Aliano
Project Manager
Christine French
Data Analyst
Carole Baggerly
Director